21-04-2022
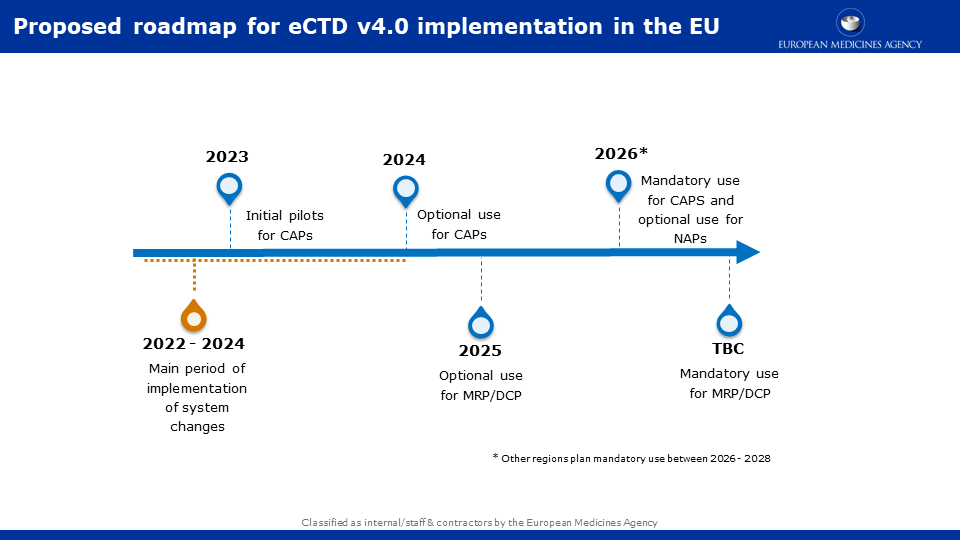
A draft timeline for the implementation of eCTD v4.0 in the EU was published in the ICH website in January 2022.
22-12-2021
Updated EU Harmonised technical eCTD guidance now available
An updated version of the EU Harmonised technical eCTD guidance and the related release notes are now available here. The updated guidance enters into force 1st February 2022.
16-09-2019
The ICH M8 Vendor Readiness Survey is now closed.
09-08-2019
Vendor Readiness Survey - NEW
The ICH M8 EWG would like to assess the readiness of eCTD v4.0 Vendors and their development and/or readiness to deliver a production ready eCTD v4.0 solution. We request that the eCTD v4.0 Vendor Readiness Survey be completed by September 15, 2019.
eCTD v4.0 Implementation package
After public consultation, discussion with ICH M8 EWG/IWG and final resolution of all comments the final version of the implementation package is now released for implementation purpose.
The package contains the updated eCTD v4.0 EU M1 Implementation Guide and the as well updated controlled vocabulary lists in machine-readable genericode format.
The package can be downloaded from the following link:
The zip folder has the following file and folder:
EU IG v1.0-20180921.pdf |
EU Module 1 eCTD Implementation Guide v1.0 |
EU_eCTDv4_0_Step5_Genericode_20180126 |
Folder of controlled vocabularies as genericode files including style sheet information |
To verify the download, the valid md5 checksum is 03a2f4a13140cb6f50f2fd9b85f0abd1.
Note: Additional samples of submissionunit.xml files will be published at a later point in time.
To support initial understanding, benefits and challenges of eCTD v4.0 implementation in the EU additional information material was developed.
In a short slide deck here the main aspects from the viewpoint of business management are demonstrated.
In a slightly extended version here also technical aspects are considered to provide a more complete overview.
The key documents for the Electronic Common Technical Document (eCTD) v4.0 Modules 2 through 5 was signed off by the ICH Steering Committee in November 2015 for ICH Step 4.
The ICH M8 Working Group on electronic submission has drafted these documents based on the Regulated Product Submission (RPS) standard established by HL7 in September 2014. This standard defines the message for exchanging regulatory submission information electronically between applicants and Regulatory Authorities.
The XML message provides the ability to describe the contents of the regulatory exchange and all information needed to process the exchange between these two parties.
The most recent version of the package of relevant documents for implementation is accessible on the ESTRI web page.
The ICH Implementation Guide and the respective set of controlled vocabularies will need to be used in conjunction with the EU Module 1 Implementation Guide and an additional set of EU relevant controlled vocabularies, as the eCTD v4.0 message will be incomplete without all of the contents.
The package from the 1st and 2nd round of public consultations can be downloaded from here.
This zip folder contains the following files:
EU IG v2.0-20150310_draft_PubCon.pdfs |
EU Module 1 eCTD Draft Implementation Guide v2.0 |
EU_eCTDv4_0_Step2_Comments_ALL.xlsx |
List of comments and their resolution in the 1st d round of public consultation |
EU IG v2.8-20170518_PubCons.pdf |
EU Module 1 eCTD Draft Implementation Guide v2.8 |
EU_eCTDv4_0_Step5_draft_v2_8_Comments IG_CV_XM-statusafter20180912 |
List of comments and their resolution in the 2nd round of public consultation |
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen
in the Protocol on Ireland/NI.
Announcement of the start of the eCTD Version 4 feasibility test
Within the context of the ICH M2 and M8 Expert Working Groups, an agreement was reached to improve the current eCTD specification.
ICH has chosen the direction of developing those standards using the HL7 messaging standard as basis for the Individual Case Safety Reporting (ICSR) as well as for eCTD. The eCTD version 4.0 will be build upon the experience gained in the first version of the Regulated Product Submission (RPS) standard. This standard defines the message for exchanging regulatory submission information electronically between applicants and Regulatory Authorities. The XML message provides the ability to describe the contents of the regulatory exchange and all information needed to process the exchange between these two parties.
Discussions at the HL7 level have been supported by the former eCTD NMV/RPS subgroup, composed of representatives of FR, NL, DE, AT, UK, IE, EMA, EFPIA, EGA, EuropaBio and AESGP.
After a positive ballot in January 2012 on the HL7 Version 3 Standard: Regulatory Product Submission Release 2 Draft Standard for Trial Use 2 the ICH M8 Expert Working Group has developed key documents for the Electronic Common Technical Document (eCTD) v4.0 Modules 2 through 5. These documents have been signed off by the ICH Steering Committee in June 2012 for ICH Step 2 for Testing. Once full documentation is available a link will be provided.
The ICH Implementation Guide and the respective set of controlled vocabularies will need to be used in conjunction with the EU Module 1 Implementation Guide and an additional set of EU relevant controlled vocabularies, as the eCTD v4.0 message will be incomplete without all of the contents.
The EU Module 1 Implementation Guide includes eCTD v4.0 Module 1 requirements of the eCTD XML message including the Regional Administrative and Product Information which is specific for EU purposes and can be downloaded via the following link:
Controlled Vocabularies relevant to EU Module 1 will be provided in a separate spreadsheet for testing purpose via the following link:
The TIGes invites all vendors of eCTD building and reviewing tools to develop tools for testing the message. ICH and TIGes are working on several test scenarios to be able to check whether previously agreed requirements are met and the message standard effectively supports the daily regulatory practice.
Any questions regarding further development, support of testing and clarifications of the released draft documents should be directed to eCTD@ema.europa.eu
Implementation in the EU
Following the publication of the ICH Implementation package, the regional Implementation Guide and related documents like the Controlled Vocabularies are being updated. A second round of public consultation is planned to take place in 2017 and will concern the EU Module 1 Implementation Guide including eCTD v4.0 Module 1 requirements of the eCTD XML message, the Regional Administrative and Product Information specific for EU purposes and the set of Controlled Vocabularies relevant to EU Module 1 in the format of genericode files.
The eSubmission Change Management Board invites all pharmaceutical companies, vendors of eCTD building and reviewing tools and competent authorities to follow up this page for news and announcements on the public consultation.
The key documents for the Electronic Common Technical Document (eCTD) v4.0 Modules 2 through 5 have been signed off by the ICH Steering Committee in December 2015 for ICH Step 4. Please also see the ESTRI web page. The ICH M8 Working Group on electronic submission has drafted these documents based on the Regulated Product Submission (RPS) standard established by HL7 in September 2014. This standard defines the message for exchanging regulatory submission information electronically between applicants and Regulatory Authorities. The XML message provides the ability to describe the contents of the regulatory exchange and all information needed to process the exchange between these two parties.
The ICH Implementation Guide and the respective set of controlled vocabularies will need to be used in conjunction with the EU Module 1 Implementation Guide and an additional set of EU relevant controlled vocabularies, as the eCTD v4.0 message will be incomplete without all of the contents.
If you have any questions regarding the development of eCTD v4.0 standard, please contact: eCTD4consultation@ema.europa.eu.
The package from the 1st round of public consultation can be downloaded here.
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland/NI.
|

